67th Annual Biosafety and Biosecurity Hybrid Conference
Scientific Program
November 3-6, 2024
All times listed will be MOUNTAIN Standard Time
Featured Speakers
Sunday, November 3, 2024
6:30 – 8:30 pm Opening Reception in the Exhibit Hall
Monday, November 4, 2024
7:00 – 5:00 pm Registration
7:00 – 8:00 am Continental Breakfast in Foyer
9:00 – 4:00 pm Vendor Exhibits
8:00 – 8:15 am Welcome and ABSA International President’s Address
Master of Ceremonies
Luis Alberto Ochoa Carrera, MSc, Michigan State University, East Lansing, MI
8:15 – 8:20 am Local Arrangements Committee Welcome
Sepideh Hockley, MBA, Penn State University, Phoenix, AZ
8:20 – 8:25 am Scientific Program Committee Welcome
AJ Troiano, PhD, RBP(ABSA), FUJIFILM Diosynth Biotechnologies, Boston, MA
Session 1 | Arnold G. Wedum Memorial Lecture Award
Introduction: Susan Vleck, PhD, RBP(ABSA), CBSP(ABSA), Stanford University, Stanford, CA
Moderator: AJ Troiano, PhD, RBP(ABSA), FUJIFILM Diosynth Biotechnologies, Boston, MA
8:25 – 9:15 am From a House on Fire to a World in Shutdown: The Intersection of Epidemiology and Vaccinology
Yvonne (Bonnie) Maldonado, MD, Stanford University School of Medicine, Palo Alto, CA
9:15 – 9:25 am Q&A Session
Moderators:
Rachel Gamble, DrPH, RBP(ABSA), CBSP(ABSA), Pond & Company, Peachtree Corners, GA
Kelly Flint, RBP(ABSA), CBSP(ABSA), SM(NRCM), National Institutes of Health, Bethesda, MD
9:55 – 10:10 am Enabling Enhanced US Made BSL-4 Ensemble
Michael Rein, PhD, Advanced Functional Fabrics of America (AFFOA), Cambridge, MA
10:10 – 10:25 am Modeling the Effects of Routine Screening for Lab–Acquired–Infections at High–Biosafety Research Facilities
Byron Cohen, PhD, Harvard University, Boston, MA
10:25 – 10:40 am The Impact of Artificial Intelligence in the Design, Construction and Operation of a Biocontainment Facility
E. Scott Kreitlein, MArch, BHDP Architects, Saratoga Springs, UT
10:40 – 10:55 am Q&A Session
10:55 – 11:00 am | Stretch
Session 3 | Perspectives on Regulations and Guidelines
Moderators:
Theresa Marth, MPH, RBP(ABSA), U.S. Food and Drug Administration, College Park, MD
Jessica McCormick-Ell, PhD, RBP(ABSA), CBSP(ABSA), National Institutes of Health, Bethesda, MD
11:00 – 11:15 am Evolving Landscape in Life Sciences Regulation: Assessing Impact and Advancing Biosafety
Randy A. Albrecht, PhD, RBP(ABSA), Icahn School of Medicine at Mount Sinai, New York, NY
11:15 – 11:30 am Using ISO 35001 as a Tool to Help Improve a Laboratory’s Local Community Relations
Jane S. Alam, MRIGlobal, Gaithersburg, MD
11:30 – 11:45 am Implementation of Global Action Plan III Laboratory Containment in Poliovirus-Essential Facilities – United States, 2017-2023
Christy Ottendorfer, PhD, Centers for Disease Control and Prevention, Atlanta, GA
11:45 – 12:00 pm Q&A Session
Session 5 | Invited Speaker
Introduction: Mike McCarthy, RBP(ABSA), Vertex Pharmaceuticals, Boston, MA
Moderator: Maya Nair, PhD, RBP(ABSA), University of North Texas Science Center, Fort Worth, TX
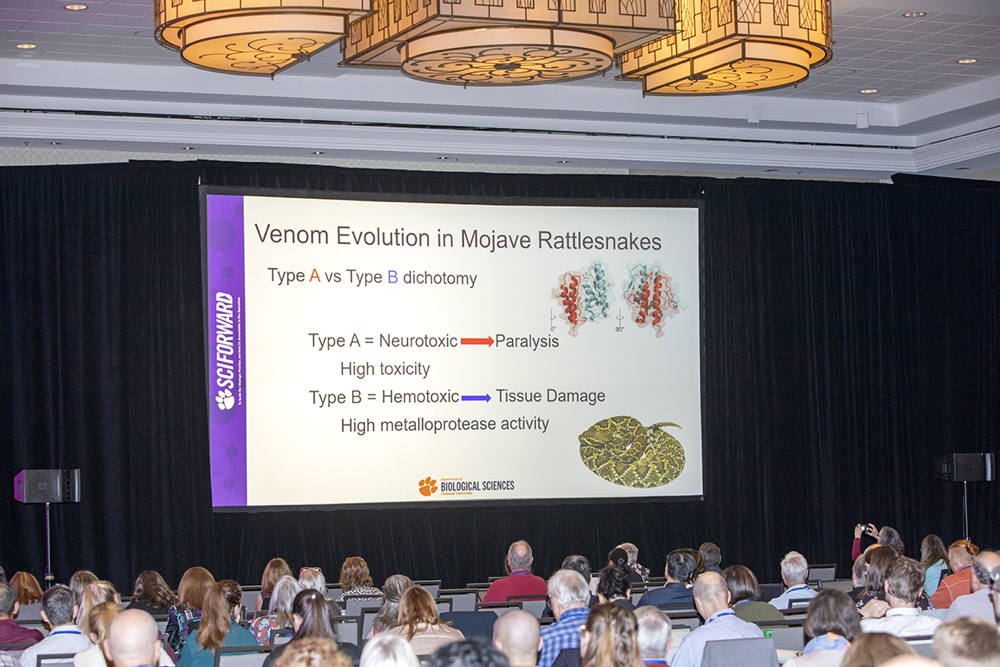
1:30 – 2:15 pm Mitigating Risk in the Field and Lab When Dealing with Venomous Creatures in Research
Christopher L. Parkinson, PhD, Clemson University, Clemson, SC
2:15 – 2:30 pm Q&A Session
Moderators:
Brett Haltiwanger, PhD, CBSP(ABSA), University of Colorado—Denver, Denver, CO
Francine Rogers, MS, RBP(ABSA), CBSP(ABSA), SM(NRCM), Tufts University, Boston, MA
3:00 – 3:15 pm Reaching Across the Bench: Why Biosafety Education is Key for Cell and Gene Therapy
Pharmacists and Clinicians
Heidi Page, MS, RBP(ABSA), SABAI Global—Shield Consulting, Chesterfield, MO
3:15 – 3:30 pm Strategies for Improving Biosafety Accessibility, Buy-In, and Compliance
Sharon Altmann, PhD, RBP(ABSA), CBSP(ABSA), MRIGlobal, Gaithersburg, MD
3:30 – 3:45 pm Establishing a Regional Training and Certification Program for BSC
Thendo Netshilema, National Institute for Communicable Diseases (NICD), Johannesburg, South Africa
3:45 – 4:00 pm Q&A Session
4:00 – 4:05 pm | Stretch
Session 7 | Clinical Biosafety
Moderators:
Colleen Kovacsics, PhD, RBP(ABSA), Sparks Therapeutics, Philadelphia, PA
Colleen Driskill, RBP(ABSA), CBSP(ABSA), University of Massachusetts Chan Medical School, Worcester, MA
4:05 – 4:20 pm Risk Assessment for Clinical Trials Involving Genetically Modified Bacteria or Phages
Daniel Eisenman, PhD, RBP(ABSA), CBSP(ABSA), SM(NRCM), Advarra, Columbia, MD
4:20 – 4:35 pm Uncovering the Hidden Threat: Acinetobacter Baumannii – A Microbiological Survey of Distribution in the Hospital Environment and Disinfectant Tolerance
Sidrah Saleem, PhD, University of Health Sciences, Lahore, Pakistan
4:35 – 4:50 pm Select Agent Regulatory Challenges in a Patient Care Setting
Corrie Ntiforo, MSPH, RBP(ABSA), University of Texas Medical Branch – Galveston, Galveston, TX
4:50 – 5:05 pm Q&A Session
5:10 – Close | Members’ Business Meeting
Door prizes will be awarded—must be present to win.
Tuesday, November 5, 2024
7:00 – 5:00 pm Registration
7:00 – 8:00 am Continental Breakfast in Foyer
9:00 – 4:00 pm Vendor Exhibits
Session 8 | Invited Speaker
Introduction: Colleen Kovacsics, PhD, RBP(ABSA), Sparks Therapeutics, Philadelphia, PA
Moderator: Adam Fleming, PhD, CBSP(ABSA), Deloitte Consulting LLP, Waldorf, MD
8:00 – 8:45 am Biosecurity: Complexity, Complacency, Connectivity and Commitment
George Poste, DVM, PhD, Arizona State University, Scottsdale, AZ
8:45 – 9:00 am Q&A Session
9:00 – 9:05 am | Stretch
Session 9 Applied Biosafety
Moderators:
Matthew Anderson, PhD, RBP(ABSA), CBSP(ABSA), MRIGlobal, Kansas City, MO
Weiying Pan, PhD, RBP(ABSA), Johns Hopkins Medicine, Baltimore, MD
9:05 – 9:20 am Deciding Upon the Best Decontamination Strategy – Follow the Literature or “Trust but Verify”?
Karen Gjendal, DVM, PhD, Bavarian Nordic A/S, Kvistgaard, Denmark
9:20 – 9:35 am Using DNA-Tagged Aerosol Tracer Particles as Challenge Agents for Measurement and Verification of Engineering Controls
Nicholas Heredia, PhD, SafeTraces, Pleasanton, CA
9:35 – 9:50 am A Modified Hand Washing Method for Resource-Limited Settings
Samreen Sarwar, MPhil, Health Security Partners, Lahore, Pakistan
9:50 – 10:05 am BSC MythBusters: Are all Performance Envelopes the Same for All BSCS?
Kara Held, PhD, Baker, Sanford, ME
10:05 – 10:35 am Q&A Session
Introduction: Mary Ann Sondrini, Eagleson Institute, Sanford, ME
Moderators:
Shelley Jones, MS, RBP(ABSA), Northern Arizona University, Flagstaff, AZ
Darlene Ward, RBP(ABSA), Advarra, Boca Raton, FL
11:05 – 11:50 am Advancing Health Knowledge with Pathogen Intelligence
David Engelthaler, PhD, Translational Genomics Research Institute and Health Observatory at ASU, Phoenix, AZ
11:50 – 12:05 am Q&A Session
Concurrent Session 12(a) | Biosafety by Design
Moderators:
Julianne Baron, PhD, RBP(ABSA), Science and Safety Consulting, Venetia, PA
Noman Siddiqi, PhD, RBP(ABSA), Harvard School of Public Health, Boston, MA
1:35 – 1:50 pm The Value of Biosafety Input at the Design Table for New or Renovated Laboratory Projects
Carrie A. Smith, PhD, RBP(ABSA), CBSP(ABSA), Merrick & Company, Greenwood Village, CO
1:50 – 2:05 pm Designing for the Future: Converting Office Space into Open Labs
Kimberly DiGiandomenico, MS, RBP(ABSA), AstraZeneca, Gaithersburg, MD
2:05 – 2:20 pm Integrating Laboratory Safety with Instrument Manufacturers
Michael Pentella, PhD, SM(ASCP), CIC, D(ABMM), State Hygienic Laboratory at the University of Iowa, Coralville, IA
2:20 – 2:35 pm Biosafety and Sustainability: Building a Resilient Future
Ryan Bartlett, MS, RBP(ABSA), CBSP(ABSA), CPBCA, Sitero, Coral Gables, FL
2:35 – 3:05 pm Q&A
Concurrent Session 12(b) | Global Perspectives
Moderators:
Michele Howard, MPH, RBP(ABSA), U.S. Food and Drug Administration, Washington, DC
Kalpana Rengarajan, PhD, MPH, JM, RBP(ABSA), Emory University, Atlanta, GA
1:35 – 1:50 pm Alternative Methods for Biohazardous Waste Inactivation
Aline Anne Baldo, DVM, PhD, Sciensano, Brussels, Belgium
1:50 – 2:05 pm Rapid Disease Screening for Biosecurity Protocols in Containment Facilities
Lia Suzanne Rotherham, PhD, Agricultural Research Council, Pretoria, South Africa
2:05 – 2:20 pm One Health Approach in the Containment of Antimicrobial Resistance: Biosafety and Biosecurity Perspectives
Md Asadulghani, PhD, International Centre of Diarrhoeal Disease Research, Bangladesh (ICDDR,B), Dhaka, Bangladesh
2:20 – 2:35 pm Establishing a Gnotobiotic Mouse Colony Disinfection Protocol
Allison Fowler, Nationwide Children’s Hospital, Columbus, OH
2:35 – 3:05 pm Q&A
Concurrent Session 13(a) | Public Health
Moderators:
Jessica McCormick–Ell, PhD, RBP(ABSA), CBSP(ABSA), National Institutes of Health, Bethesda, MD
Aderemi Dosunmu, PhD, CBSP(ABSA), Mount Sinai, New York, NY
4:05 4:20 pm Biorisk Management Implementation in Select Public Health Laboratories Across the U.S.
Folasade Kembi, PhD, CPH, MPH, MSc, Centers for Disease Control and Prevention, Atlanta, GA
4:20 – 4:35 pm Advancing Health and Safety at the NICD in Johannesburg, South Africa
Kovashnee Naidoo, PhD, National Institute for Communicable Diseases (NICD), Johannesburg, South Africa
4:35 – 4:50 pm Designing and Validating a Framework for Multisectoral Preparedness for Deliberate Biological Incidents Involving Zoonotic Pathogens in Egypt
Ali M. Asy, PhD, Animal Health Research Institute, Benha, Egypt
4:50 –5:05 pm Q&A
Concurrent Session 13(b) | High Containment Case Studies
Moderators:
Kaci Van Dalen, MS, NIH/NIAID, Bethesda, MD
Max Schroeder, PhD, RBP(ABSA), Centers for Disease Control and Prevention, Atlanta, GA
4:05 4:20 pm Adapting Small Animal MRI for Safe Use in BSL-3 Facilities
Alexander R. Kneubehl, PhD, Baylor College of Medicine, Houston, TX
4:20 – 4:35 pm Establishing a High Containment Laboratory in the Resource-Limited Country
Angelo Dela Tonga, MSc, MTM, University of the Philippines Manila, Manila, Philippines
4:35 – 4:50 pm A Case Study of the Complexities with Finalizing ABSL-3 Construction Project
Beata Clapp, DVM, PhD, Penn State University, University Park, PA
4:50 –5:05 pm Q&A
6:00 – 10:00 pm | Banquet at Desert Foothills

 This year's banquet will take place at Desert Foothills, a breathtaking private events venue located in North Scottsdale, just a 10-minute drive from the JW Marriott Desert Ridge Resort and Spa conference site. Selected for its exquisite views of the Sonoran Desert, set against the backdrop of the Valley of the Sun Phoenix Metro area, this venue showcases the unique beauty of the region. The Sonoran Desert stands out as one of the most ecologically diverse deserts in North America, boasting a wide array of plant and animal life. As you take in the scenery, savor the food and drinks, and partake in games, music, and the warmth of fire pits. Join us for a delightful evening of great barbeque, entertainment, and stunning landscapes with friends and colleagues.
This year's banquet will take place at Desert Foothills, a breathtaking private events venue located in North Scottsdale, just a 10-minute drive from the JW Marriott Desert Ridge Resort and Spa conference site. Selected for its exquisite views of the Sonoran Desert, set against the backdrop of the Valley of the Sun Phoenix Metro area, this venue showcases the unique beauty of the region. The Sonoran Desert stands out as one of the most ecologically diverse deserts in North America, boasting a wide array of plant and animal life. As you take in the scenery, savor the food and drinks, and partake in games, music, and the warmth of fire pits. Join us for a delightful evening of great barbeque, entertainment, and stunning landscapes with friends and colleagues.
Wednesday, November 6, 2024
7:00 – 5:00 pm Registration
7:00 – 8:00 am Continental Breakfast in Foyer
Session 14 | Griffin Lecture Award
Introduction: Erin Sorrell, PhD, MSc, Elizabeth R. Griffin Program, Washington, DC
Moderators: Sarah Ziegler, PhD, RBP(ABSA), CBSP(ABSA), SM(NRCM), Sitero, Coral Gables, FL
8:00 – 8:45 am Strengthening National and Regional Collaboration for Laboratory Biorisk Management in Mali and Across West and Central Africa
Djibril Sangaré, PhD, Malaria Research and Training Center (MRTC), Bamako, Mali
8:45 – 9:00 am Q&A Session
9:00 – 9:05 am | Stretch
Session 15 | Biosafety Program Management: Topics, Trends, and Analyses
Moderators:
Sunny Hoffman, MPH, RBP(ABSA), Clinical Biosafety Services, Columbia, MO
James Baugh, PhD, CBSP(ABSA), SM(NRCM), University of California—Berkeley, Berkeley, CA
9:05 – 9:20 am Biosafety Assurance: Tracking Compliance and Operational Excellence
Luis Ochoa Carrera, MSc, Michigan State University, Lansing, MI
9:20 – 9:35 am Trends in Demographics Among Biosafety Professionals: A Decade-Long Analysis
An Tran, University of Nevada, Reno, Reno, NV
9:35 – 9:50 am Developing an Effort Coefficient to Strengthen Programmatic Statistics
Andrew B. Maksymowych, PhD, MS, RBP(ABSA), University of Pennsylvania, Philadelphia, PA
9:50 – 10:05 am The Impact of Targeted Interventions on Overall Safety Culture and Injuries
Samantha Parsons, Nationwide Children’s Hospital, Columbus, OH
10:05 – 10:35 am Q&A Session
10:35 – 10:45 am | Coffee Break
Session 16 | Animal Biosafety
Moderators:
Gregory Powell, PhD, RBP(ABSA), Arizona State University, Tempe, AZ
Frank Novembre, PhD, RBP(ABSA), Florida Atlantic University, Boca Raton, FL
10:45 – 11:00 am Improving Hazard Communication in Animal Research
Sarah Capasso, PhD, RBP(ABSA), University of Pennsylvania, Philadelphia, PA
11:00 – 11:15 am Adapting a Behavioral Arena for Rodent Neuroinfectious Disease Research
Brinkley Morse, Baylor College of Medicine, Houston, TX
11:15 – 11:30 am The Introduction of University of Southern California’s Newly Established Animal Research Safety Program Within its Research Community
Bagrat Khachatryan, PhD, University of Southern California, Los Angeles, CA
11:30 – 11:45 am Expecting the Unexpected – Preparations and Complications of an ABSL-3 Renovation Project
Liliam Montoya, University of California – Berkeley, Berkeley, CA
11:45 – 12:15 pm Q&A
12:15 – 1:45 pm | Honor Awards and Special Recognition Luncheon
Presenter: Luis Alberto Ochoa Carrera, MSc, Michigan State University, East Lansing, MI
Arnold G. Wedum Distinguished Achievement Award
Everett J. Hanel, Jr. Presidential Award
John H. Richardson Special Recognition Award
Diane Fleming Leadership Award
Scientific and Informational Poster Awards
Hashimoto Award for Service and Honor
Richard Rebar Recognition of Certified Biological Safety Professionals and Registered Biosafety Professionals
Session 17 | Richard Knudsen Award
Introduction: Matthew Anderson, PhD, RBP(ABSA), CBSP(ABSA), MRIGlobal, Kansas City, MO
Moderator: Darlene Ward, RBP(ABSA), Advarra, Boca Raton, FL
1:45 – 2:05 pm An Assessment of Germicidal Ultraviolet Treatment Cabinets and Carousels Using a Bacteriophage Surface Challenge
Alan Beswick, PhD, Health and Safety Executive Science and Research Centre, Buxton, United Kingdom
2:05 – 2:15 pm Q&A Session
2:15 – 2:20 pm | Stretch
Session 18 | Navigating Challenges in Biosafety Facilities
Moderators:
Shelley Jones, MS, RBP(ABSA), Northern Arizona University, Flagstaff, AZ
Jeffrey Potts, MPH, CBSP(ABSA), U.S. Geological Survey, Falls Church, VA
2:20 – 2:35 pm Fixing Broken Laboratories
Mark Wheatley, BEng, CEng, MCIBSE, Environment & Services Ltd., Cornwall, United Kingdom
2:35 – 2:50 pm BSL-2 Lab Decommissioning Project Management: Challenges and Solutions
Jacqueline Hardt, CBSP(ABSA), Zoubek Consulting LLC, Palm Springs, CA
2:50 – 3:05 pm The Cold Room That We Forgot
Tim Strovas, PhD, RBP(ABSA), Puget Sound Veteran Affairs Medical Center, Seattle, WA
3:05 – 3:20 pm Air Changes Effectiveness in Laboratories
Cory Ziegler, PE, Merrick & Company, Greenwood Village, CO
3:20 – 3:50 pm Q&A
3:50 – 4:00 pm | Coffee Break
Session 19 | Biosecurity
Moderators:
Susan Vleck, PhD, RBP(ABSA), CBSP(ABSA), Stanford University, Stanford, CA
Brett Haltiwanger, PhD, CBSP(ABSA), University of Colorado—Denver, Denver, CO
4:00 – 4:15 pm Robert I. Gross Student Award: International Biosecurity Education Network: A Timely Tool For Biosecurity
Iris Magne, London Metropolitan University, London, England
4:15 – 4:30 pm Biosecurity Risk Assessment for the Use of Artificial Intelligence in Synthetic Biology: A Practical Approach
Leyma P. De Haro, PhD, RBP(ABSA), Merrick & Company, Greenwood Village, CO
4:30 – 4:45 pm A Century of Assessment – The Collection of Biothreat Risk Assessment (COBRA)
Michael Parker, PhD, Georgetown University, Washington, DC
4:45 – 5:00 pm Q&A Session
5:00 pm | Close of Conference
Sherry Bohn, PhD, CBSP(ABSA), SM(NRCM), University of Maryland—Baltimore, Baltimore, MD