TUESDAY SCHEDULE:
Scientific Program
October 28, 2025
All times listed will be Eastern Standard Time
7:00 am – 5:00 pm Registration
7:00 – 8:00 am Continental Breakfast in Foyer
9:00 am – 3:35 pm Vendor Exhibits
Special Presentation
7:40 – 8:00 am Update from NIH: Modernizing and Strengthening Biosafety Oversight
Cari Young, ScM, National Institutes of Health, Bethesda, MD
Session 8 | Eagleson Lecture Award
Introduction: Mary Ann Sondrini, Eagleson Institute, Sanford, ME
Moderator: Susan Vleck, PhD, RBP(ABSA), CBSP(ABSA), Stanford University, Stanford, CA
8:00 – 8:45 am Special Challenges in the Development of Interventions Against Highly Pathogenic Avian Influenza Viruses
Nicholas Heaton, PhD, Duke University, Durham, NC
8:45 – 9:00 am Q&A Session
9:00 – 9:05 am | Stretch
Session 9 Navigating Regulations
Moderators:
Colleen Driskill, RBP(ABSA), CBSP(ABSA), University of Massachusetts Chan Medical School, Worcester, MA
Betsy Matos, PhD, CBSP(ABSA), Iowa State University, Ames, IA
9:05 – 9:20 am New International Labour Organization (ILO) Biological Hazard Standard: A Milestone in Global Occupational Safety
Bria Kettle, PhD, RBP(ABSA), Bayer, Chesterfield, MO
9:20 – 9:35 am Renewed European Standard for BSCs–Impact and Comparison to NSF/ANSI 49
Thomas Hinrichs, Berner International, Elmshorn, Germany
9:35 – 9:50 am An Entity’s Journey in Building a Select Agent Program with No Prior Experience
Endah Sulistijo, PhD, RBP(ABSA), CBSP(ABSA), Temple University, Philadelphia, PA
9:50 – 10:05 am Q&A Session
10:05 – 10:35 am | Exhibits, Posters, and Coffee Break
Session 10 |Invited Speaker
Introduction: AJ Troiano, PhD, RBP(ABSA), FUJIFILM Diosynth Biotechnologies, Boston, MA
Moderator: Don Sibley, PhD, Cepheid, Sunnyvale, CA
10:35 – 11:20 am Immunotherapy in the Brain with Recombinant Poliovirus
Matthias Gromeier, MD, PhD, Duke University School of Medicine, Durham, NC
11:20 – 11:35 am Q&A Session
11:35 am – 1:05 pm | Exhibits, Posters, and Lunch
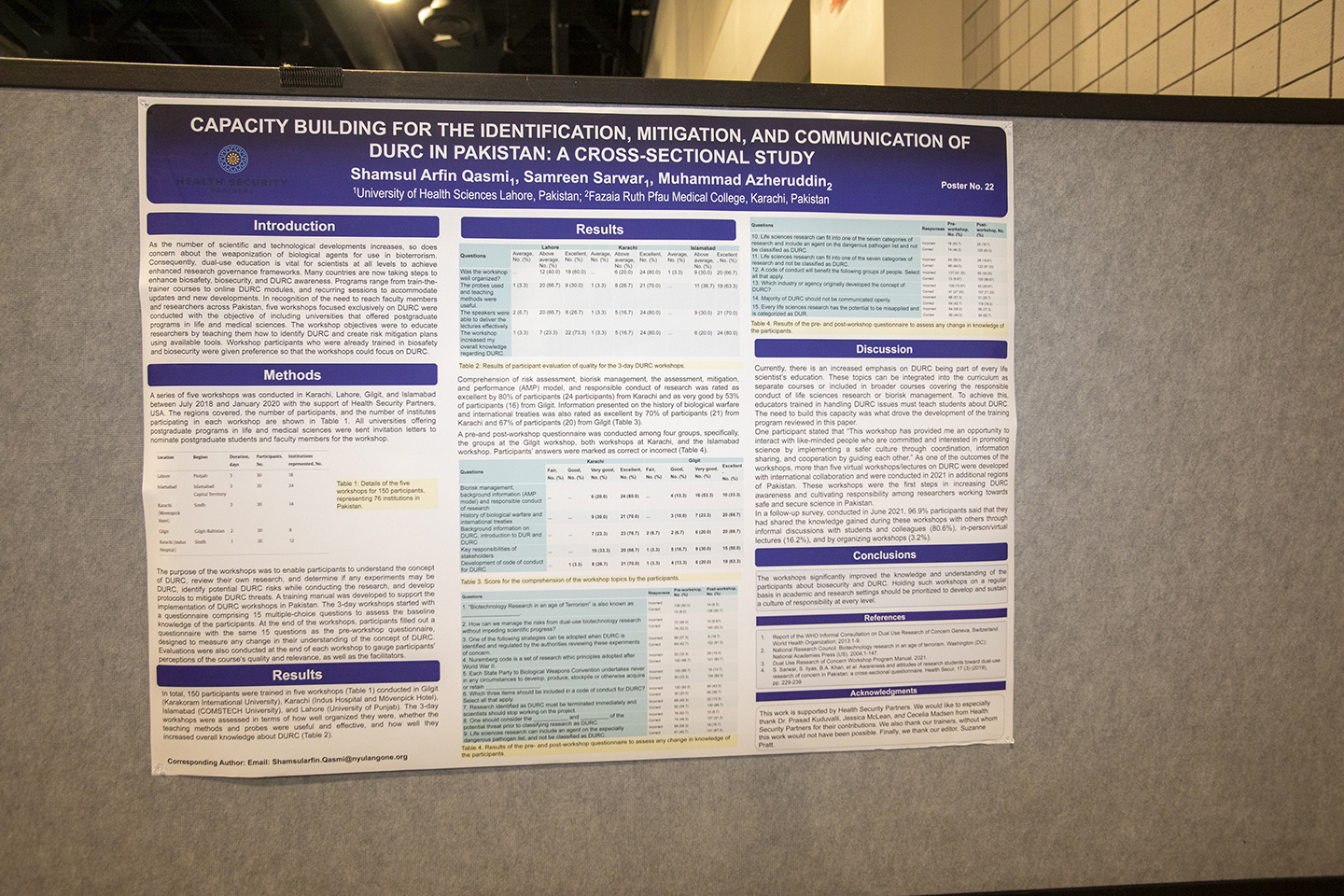
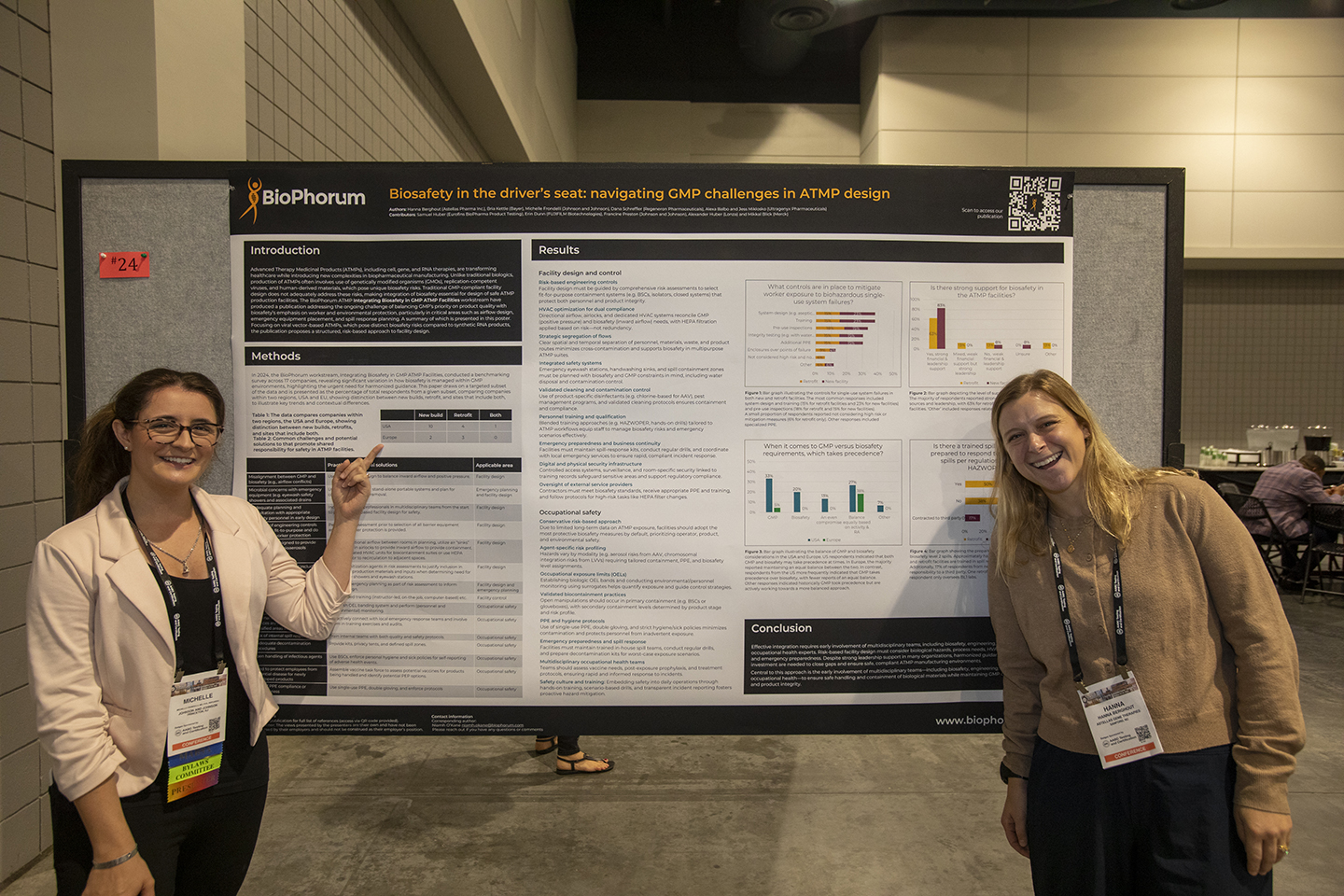
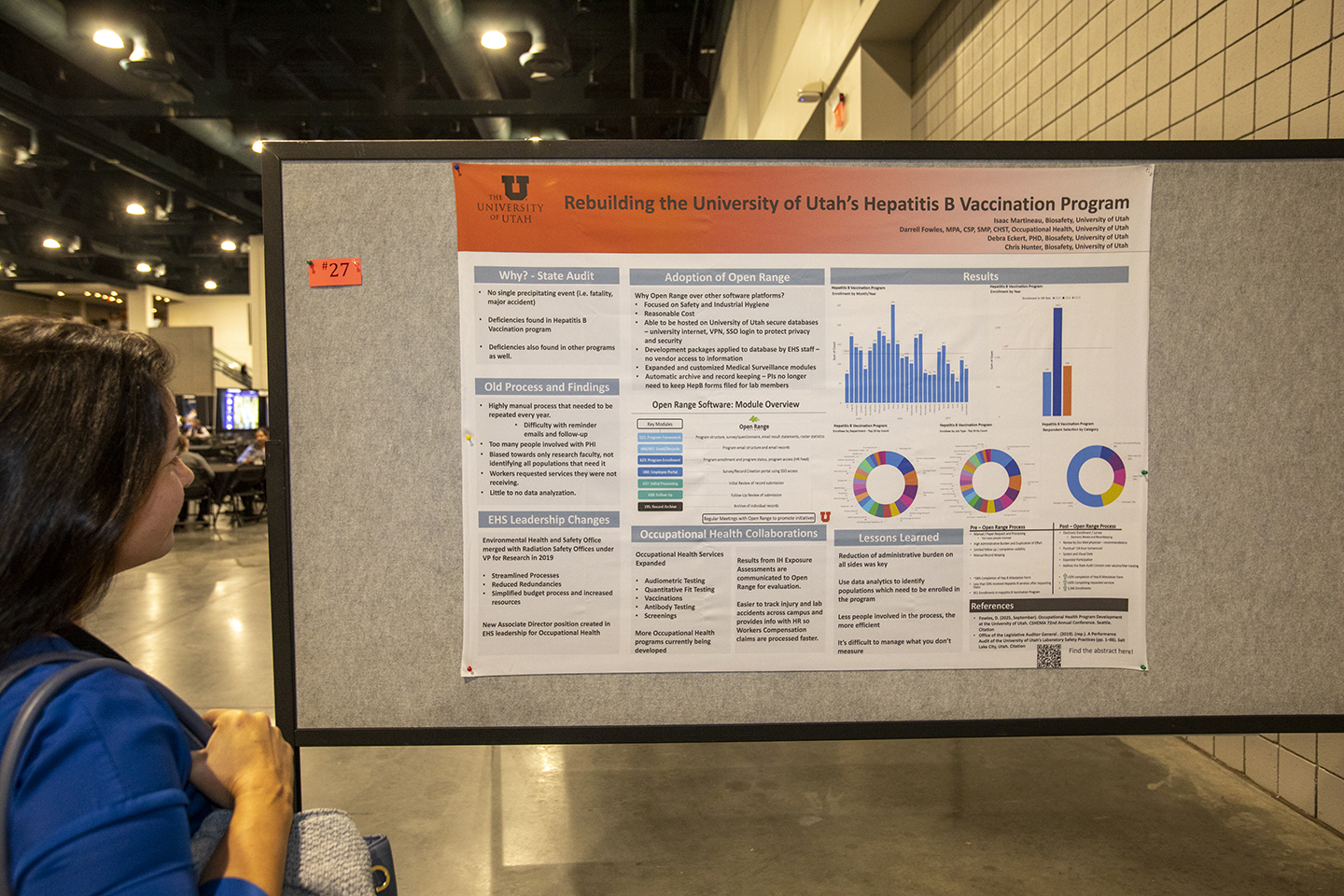
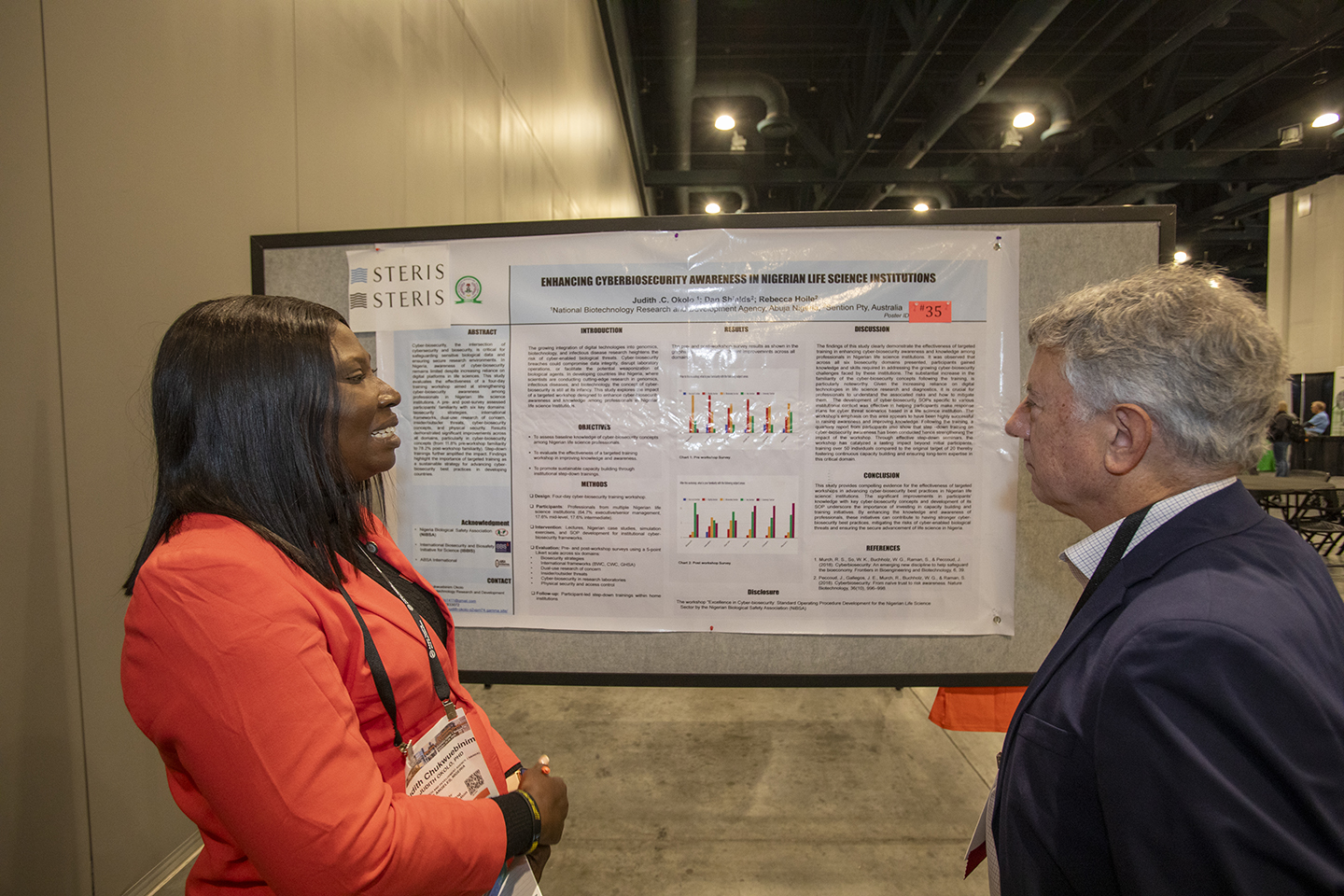
Session 11 | Poster Session
12:05 – 1:05 pm Presenters must be available during the session.
Concurrent Session 12(a) | High-Containment
Moderators:
Matthew Anderson, PhD, RBP(ABSA), CBSP(ABSA), MRIGlobal, Kansas City, MO
Kaci Van Dalen, MS, National Institutes of Health—NIAID, Bethesda, MD
1:05 – 1:20 pm Hello Again: Reestablishing BSL-3 Laboratories by Enhancing Safety and Research Potential
Bárbara Ponzilacqua-Silva, DVM, PhD, University of Chicago, Chicago, IL
1:20 – 1:35 pm Harmonization Effort to Enhance Compliance in High-Containment Environments
Andrea Vogel, PhD, CBSP(ABSA), Duke University Health System, Durham, NC
1:35 – 1:50 pm Biorisk Upgrades at INRB and Regional Collaboration—Democratic Republic of the Congo
Valentin Disashi N., MEng, National Institute of Biomedical Research, Kinshasa, Democratic Republic of the Congo
1:50 – 2:05 pm Bridging the Biosafety Knowledge Gap Through Essential BSL-3 Training
Haley DeMers, PhD, RBP(ABSA), University of California—Irvine, Irvine, CA
2:05 – 2:35 pm Q&A Session
Concurrent Session 12(b) | Technology and Biosafety
Moderators:
Darlene Ward, RBP(ABSA), Advarra, Boca Raton, FL
Frank Novembre, PhD, RBP(ABSA), Florida Atlantic University, Boca Raton, FL
1:05 – 1:20 pm Using Embodied AI Agents to Automate High-Containment Laboratories
Leyma De Haro, PhD, RBP(ABSA), RBSO(CABS), Merrick & Company, Greenwood Village, CO
1:20 – 1:35 pm Empowering Non-Coders to Automate Biosafety Audit Analysis Using Open-Source Tools and AI Support
Marina Kirkland, PhD, RBP(ABSA), Dana-Farber Cancer Institute, Boston, MA
1:35 – 1:50 pm Lab Biorisk Assessments: Considerations for Incorporating Cyberbiosecurity
Julianne Baron, PhD, RBP(ABSA), CPH, Science and Safety Consulting, McMurray, PA
1:50 – 2:05 pm CMMC Compliance for Laboratory Companies: Safeguarding Sensitive Data in Research and Testing
Daniel Tuten, PhD, King Tiger Consulting LLC, Stone Mountain, GA
2:05 – 2:35 pm Q&A Session
2:35 – 3:35 pm | Coffee Break, Posters, Exhibits, and Raffle
Exhibitor raffle prized will be awarded – must be present to win
Concurrent Session 13(a) | Global Innovation
Moderators:
Maya Nair, PhD, RBP(ABSA), University of North Texas Science Center, Fort Worth, TX
Adam Fleming, PhD, CBSP(ABSA), Deloitte Consulting, LLP, Waldorf, MD
3:35 – 3:50 pm Sustainable Biomedical Waste Management in Bangladesh: A Decade Long Success Story
Natasha Griffith, MS, RBP(ABSA), SOTER Bio Consulting, Atlanta, GA
3:50 – 4:05 pm One Health Approach- Expanding High-Containment Research Capacity in Taiwan
Wen-Chun Liu, PhD, Academia Sinica, Taipei City, Taiwan
4:05 – 4:20 pm Project Orion–Merging Physics and Microbiology in Brazil
Robert Mills, MArch, WSP USA Inc., Atlanta, GA
4:20 – 4:35 pm Q&A Session
Concurrent Session 13(b) | Engaging Future and Current BSOs
Moderators:
Julianne Baron, PhD, RBP(ABSA), Science and Safety Consulting, Venetia, PA
Kalpana Rengarajan, PhD, MPH, JM, RBP(ABSA), Emory University, Atlanta, GA
3:35 – 3:50 pm Specialized Certificate Development: Introduction to Biosafety as a Career
Cindy Trigueros, MHA, University of California—Irvine, Irvine, CA
3:50 – 4:05 pm Empowering the Next Generation: Impact of Biosafety and Biosecurity Education Among High School Students in Nigeria
Judith Okolo, PhD, National Biotechnology Research and Development Agency, Lugbe, Abuja, Nigeria
4:05 – 4:20 pm Strengthening Biosafety Workforce Through Community Engagement
Stormy Chester, Association of Public Health Laboratories, Bethesda, MD
4:20 – 4:35 pm Q&A Session
6:00 – 10:00 pm | Banquet at North Carolina Museum of Natural Sciences

 This year’s banquet will take place at the North Carolina Museum of Natural Sciences in Raleigh!
This year’s banquet will take place at the North Carolina Museum of Natural Sciences in Raleigh!
It will be an evening of music, great food, and lively conversation set in one of the area’s most remarkable venues. Enjoy the chance to explore captivating exhibits, from giant dinosaurs to interactive science displays, as we gather for a night of entertainment. Whether dining under a whale skeleton or strolling through a tropical forest, there’s something for everyone to enjoy. Don’t miss this special night of celebration and discovery, a true highlight of the week.